37629-51-9
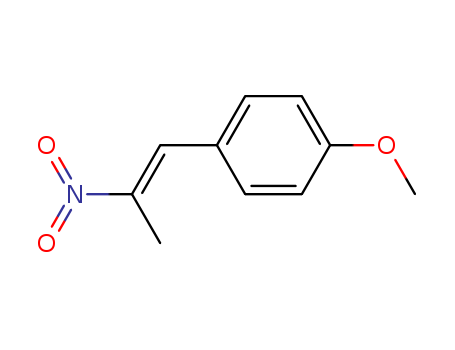
- Product Name:1-(4-METHOXYPHENYL)-2-NITROPROPENE
- Molecular Formula:C10H11NO3
- Purity:99%
- Molecular Weight:193.202
Product Details;
CasNo: 37629-51-9
Molecular Formula: C10H11NO3
Quality Manufacturer Supply 99% Pure 1-(4-METHOXYPHENYL)-2-NITROPROPENE 37629-51-9 Low Price
- Molecular Formula:C10H11NO3
- Molecular Weight:193.202
- Vapor Pressure:0.000551mmHg at 25°C
- Boiling Point:321.7oC at 760 mmHg
- Flash Point:147.4oC
- PSA:55.05000
- Density:1.156g/cm3
- LogP:2.85590
1-(4-METHOXYPHENYL)-2-NITROPROPENE(Cas 37629-51-9) Usage
1-(4-methoxyphenyl)-2-nitropropene is non-planar. These chemicals, including 1-(4-methoxyphenyl)-2-nitropropene, are utilized in various applications, ranging from organic synthesis to the pharmaceutical industry.
InChI:InChI=1/C10H11NO3/c1-8(11(12)13)7-9-3-5-10(14-2)6-4-9/h3-7H,1-2H3/b8-7+
37629-51-9 Relevant articles
Discovery of Cytochrome P450 4F11 Activated Inhibitors of Stearoyl Coenzyme A Desaturase
Winterton, Sarah E.,Capota, Emanuela,Wang, Xiaoyu,Chen, Hong,Mallipeddi, Prema L.,Williams, Noelle S.,Posner, Bruce A.,Nijhawan, Deepak,Ready, Joseph M.
, p. 5199 - 5221 (2018/06/13)
Stearoyl-CoA desaturase (SCD) catalyzes ...
Catalyst- and Substituent-Controlled Switching of Chemoselectivity for the Enantioselective Synthesis of Fully Substituted Cyclobutane Derivatives via 2 + 2 Annulation of Vinylogous Ketone Enolates and Nitroalkene
Akula, Pavan Sudheer,Hong, Bor-Cherng,Lee, Gene-Hsiang
supporting information, p. 7835 - 7839 (2019/01/04)
The first regioselective, diastereoselec...
Catalytic asymmetric Tamura cycloadditions involving nitroalkenes
Manoni, Francesco,Farid, Umar,Trujillo, Cristina,Connon, Stephen J.
, p. 1463 - 1474 (2017/02/15)
The first examples of asymmetric Tamura ...
37629-51-9 Process route
-
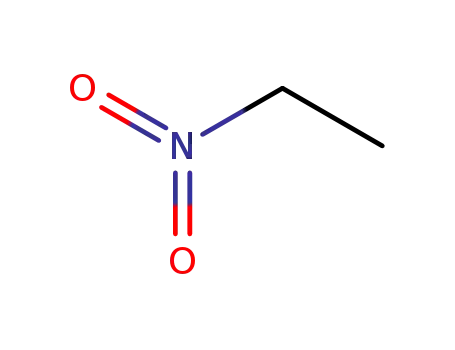
- 79-24-3
Nitroethane

-
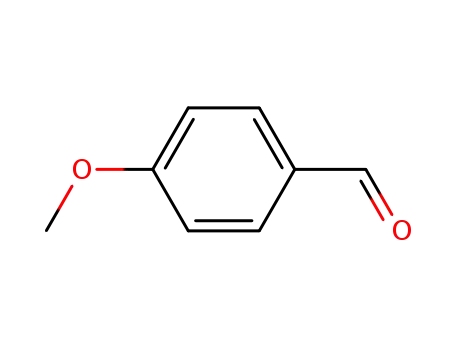
- 123-11-5
4-methoxy-benzaldehyde

-
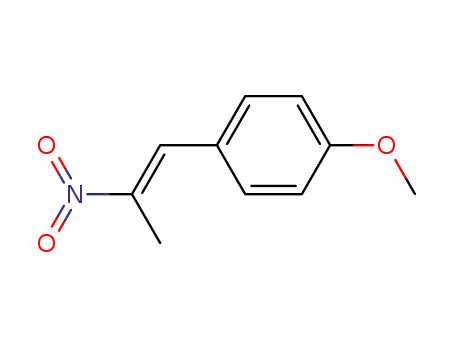
- 37629-51-9
1-methoxy-4-((E)-2-nitroprop-1-enyl)benzene
| Conditions | Yield |
|---|---|
|
With ammonium acetate; In toluene; at 100 ℃; for 22h;
|
99% |
|
MCM-41-NH2; at 90 ℃; for 6h;
|
95% |
|
With piperidine; iron(III) chloride; In toluene; for 6h; Reflux;
|
94% |
|
aminopropyl-functionalized silica; at 110 ℃; for 5h;
|
92% |
|
With phenolformaldehyde-based ethylenediamine-modified polymer; at 90 ℃; for 6h;
|
84% |
|
With ammonium acetate; for 0.583333h; Heating;
|
81% |
|
With O-(tert-butyldiphenylsilyl)-L-tyrosine lithium salt; magnesium sulfate; In dichloromethane; at 25 ℃; for 96h; stereoselective reaction;
|
81% |
|
With isobutylamine; In acetic acid; at 50 ℃; for 3h; under 7500600 Torr;
|
80% |
|
morpholine on silica gel; In acetonitrile; at 25 ℃; for 0.1h;
|
78% |
|
With ammonium acetate; at 60 ℃; for 4h;
|
76% |
|
With ammonium acetate; at 120 - 130 ℃; for 2h;
|
73% |
|
With ammonium acetate; for 2h; Heating;
|
71% |
|
With N-butylamine; In toluene; for 8h; Reflux;
|
66% |
|
With CsNaX zeolite; for 24h; Heating;
|
65% |
|
Nitroethane; 4-methoxy-benzaldehyde; With potassium tert-butylate; In tetrahydrofuran; tert-butyl alcohol; at 0 - 20 ℃; for 12h; Inert atmosphere;
With trifluoroacetic anhydride; In dichloromethane; at -10 ℃; for 0.5h; Inert atmosphere;
With triethylamine; In dichloromethane; at -10 ℃; for 0.5h; Inert atmosphere;
|
65% |
|
With ammonium acetate; at 110 ℃; for 45h; Inert atmosphere;
|
49.5% |
|
With ammonium acetate; Heating;
|
48% |
|
With n-Pentylamine; at 20 ℃;
|
|
|
With ethanol; N-butylamine;
|
|
|
With ammonium acetate; acetic acid;
|
|
|
|
|
|
With propylamine; Ambient temperature;
|
|
|
|
|
|
With ammonium acetate; for 2h; Inert atmosphere; Reflux;
|
|
|
With acetic acid; N-butylamine; at 60 ℃; Sonication;
|
-
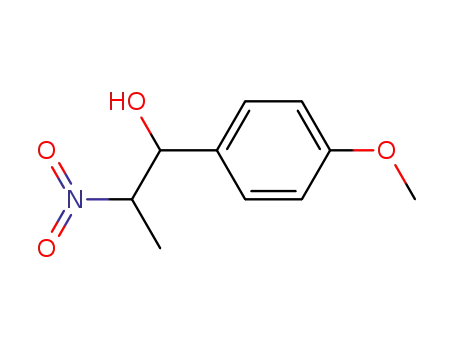
- 148527-33-7
2-nitro-1-hydroxy-1-(4-methoxy-phenyl)-propane

-

- 37629-51-9
1-methoxy-4-((E)-2-nitroprop-1-enyl)benzene
| Conditions | Yield |
|---|---|
|
With triethylamine; triphenylphosphine; In tetrachloromethane; for 2h; Heating;
|
95% |
|
Multi-step reaction with 2 steps
1: CH2Cl2
2: NEt3 / CH2Cl2
With triethylamine; In dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: pyridine / 2 h / Ambient temperature
2: sodium carbonate / benzene / 5 h / Heating
With pyridine; sodium carbonate; In benzene;
|
|
|
With 1H-imidazole; iodine; In dichloromethane; for 0.833333h; Reagent/catalyst;
|
37629-51-9 Upstream products
-
148527-35-9
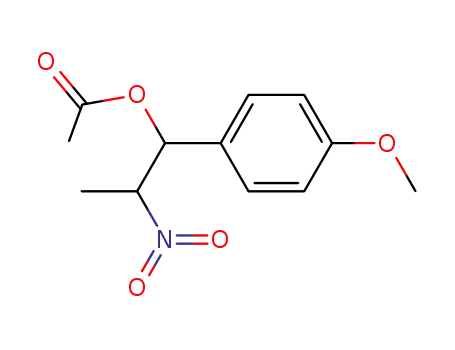
1-(4-methoxyphenyl)-2-nitropropyl acetate
-
79-24-3

Nitroethane
-
123-11-5

4-methoxy-benzaldehyde
-
148527-33-7

2-nitro-1-hydroxy-1-(4-methoxy-phenyl)-propane
37629-51-9 Downstream products
-
64-13-1
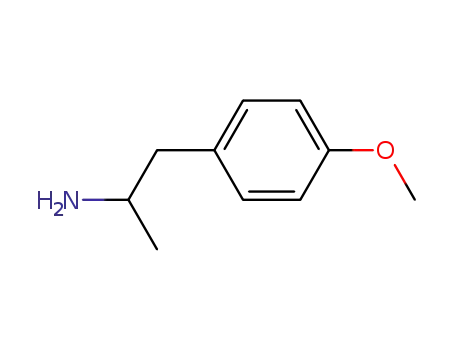
2-amino-1-(4-methyoxyphenyl)propane
-
89447-00-7
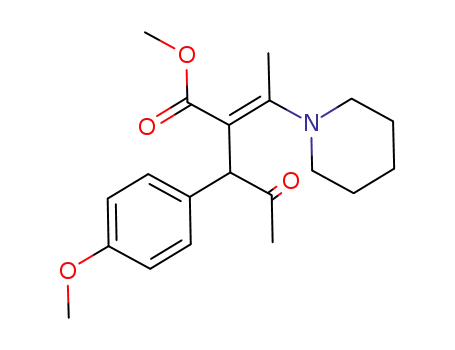
3-(4-Methoxy-phenyl)-4-oxo-2-[1-piperidin-1-yl-eth-(E)-ylidene]-pentanoic acid methyl ester
-
51463-84-4
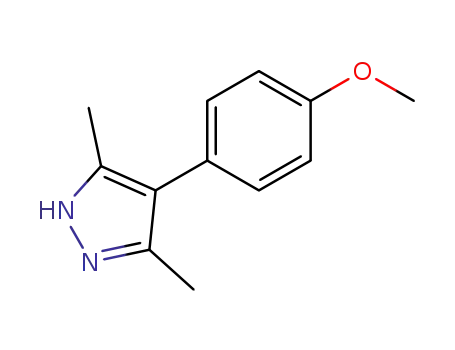
4-(4-Methoxy-phenyl)-3,5-dimethyl-1H-pyrazole
-
120290-04-2
2,4-dinitro-3-(4'-methoxyphenyl)pentane
Relevant Products
-
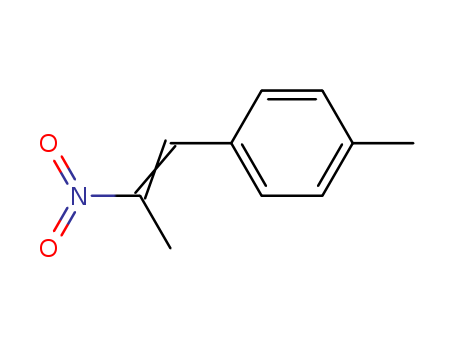
1-(4-Methylphenyl)-2-nitropropene
CAS:29816-55-5
-
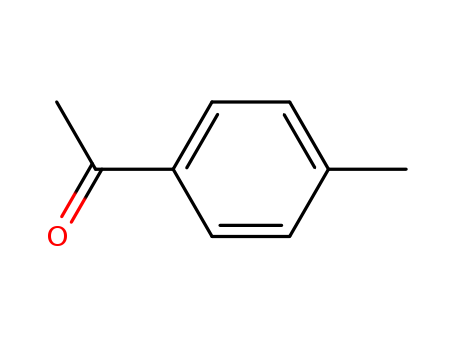
4'-Methylacetophenone
CAS:122-00-9
-
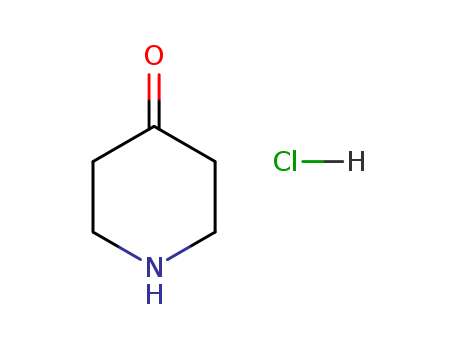
4-oxopiperidinium chloride
CAS:41979-39-9