87120-72-7
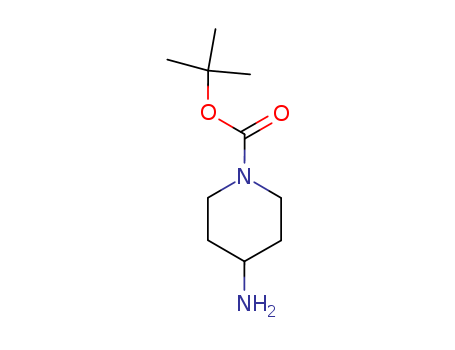
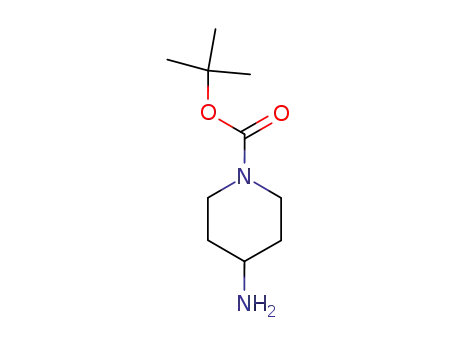
- Product Name:4-Amino-1-Boc-piperidine
- Molecular Formula:C10H20N2O2
- Purity:99%
- Molecular Weight:200.281
Product Details;
CasNo: 87120-72-7
Molecular Formula: C10H20N2O2
Appearance: white to light yellow crystal powder
Buy High Quality Top Purity 99% 4-Amino-1-Boc-piperidine 87120-72-7 Customized Supply
- Molecular Formula:C10H20N2O2
- Molecular Weight:200.281
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.00456mmHg at 25°C
- Melting Point:50 °C
- Boiling Point:277.3 °C at 760 mmHg
- PKA:10.10±0.20(Predicted)
- Flash Point:121.5 °C
- PSA:55.56000
- Density:1.041 g/cm3
- LogP:1.98280
4-Amino-1-Boc-piperidine(Cas 87120-72-7) Usage
|
Chemical Properties |
white to light yellow crystal powder |
|
Uses |
4-Amino-1-Boc-piperidine is a chemical reagent used in the preparation of pharmaceutical compounds. Used in the synthesis of bromodomain inhibitors as well as HepG2 cell cycle inhibitors used in anti-tumor therapy. Also employed in a microwave-assisted solid-phase synthesis of N-substituted piperidines via direct annulation of primary amines with resin-bound dimesylates. |
InChI:InChI=1/C10H20N2O2/c1-10(2,3)14-9(13)12-6-4-8(11)5-7-12/h8H,4-7,11H2,1-3H3/p+1
87120-72-7 Relevant articles
Copper-catalysed amination of alkyl iodides enabled by halogen-atom transfer
Barthelemy, Anne-Laure,Douglas, James J.,Górski, Bartosz,Juliá, Fabio,Leonori, Daniele
, p. 623 - 630 (2021/07/25)
Despite the fact that nucleophilic displ...
sEH Inhibitor or pharmaceutically acceptable composition thereof as well as preparation method and application thereof
-
Paragraph 0117; 0119, (2021/09/21)
The invention provides sEH inhibitor or ...
Direct Deamination of Primary Amines via Isodiazene Intermediates
Berger, Kathleen J.,Driscoll, Julia L.,Yuan, Mingbin,Dherange, Balu D.,Gutierrez, Osvaldo,Levin, Mark D.
supporting information, p. 17366 - 17373 (2021/11/04)
We report here a reaction that selective...
PROCESSES AND COMPOUNDS FOR THE DECARBOXYLATIVE AMINATION OF REDOX-ACTIVE ESTERS WITH DIAZIRINES
-
Page/Page column 38, (2020/12/30)
The invention described herein relates g...
87120-72-7 Process route
-
-
C18H24F3N3O2

-
-
434-45-7
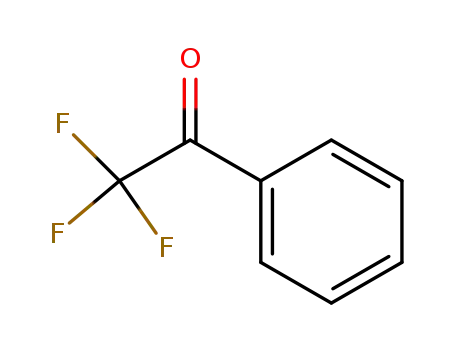
2,2,2-Trifluoroacetophenone

-
-
87120-72-7
1-(tert-butoxycarbonyl)-4-aminopiperidine
| Conditions | Yield |
|---|---|
|
With
chloro-trimethyl-silane; lithium chloride;
|
90% |
-
-
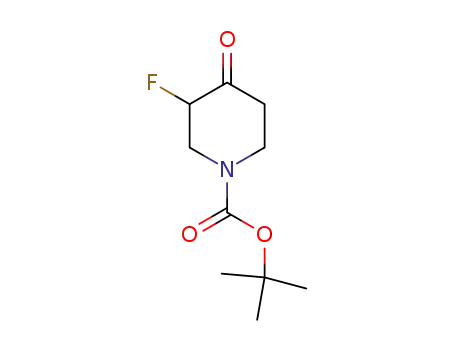
211108-50-8
tert-butyl-3-fluoro-4-oxopiperidine-1-carboxylate

-
-
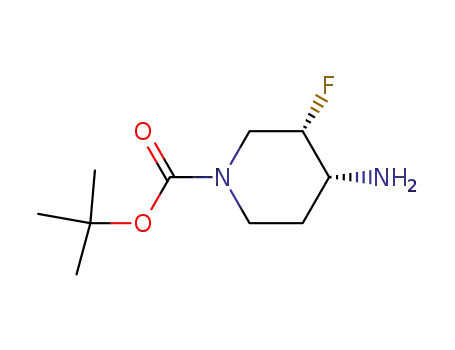
907544-20-1,577691-56-6
(3S,4R)-tert-butyl 4-amino-3-fluoropiperidine-1-carboxylate

-
-
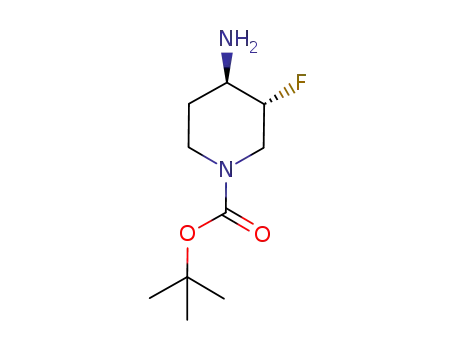
1260612-08-5,577691-56-6
(3R,4R)-tert-butyl 4-amino-3-fluoropiperidine-1-carboxylate

-
-
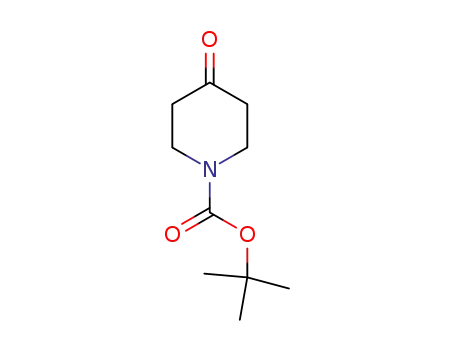
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-

-
87120-72-7
1-(tert-butoxycarbonyl)-4-aminopiperidine
| Conditions | Yield |
|---|---|
|
With
sodium tetraborate decahydrate; pyridoxal 5'-phosphate; Codexis ATA-303 transaminase; isopropylamine;
In
water; dimethyl sulfoxide;
at 20 - 45 ℃;
for 24h;
pH=10.5;
pH-value;
enantioselective reaction;
Inert atmosphere;
Enzymatic reaction;
|
94 % ee |
87120-72-7 Upstream products
-
153198-06-2
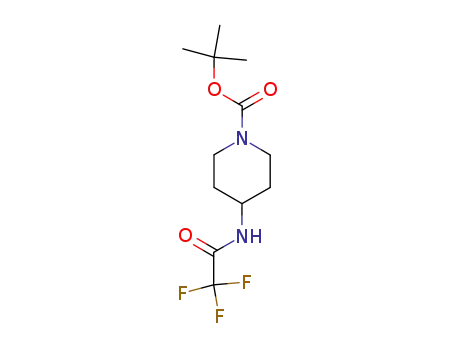
tert-butyl 4-(2,2,2-trifluoroacetamido)piperidine-1-carboxylate
-
180695-80-1
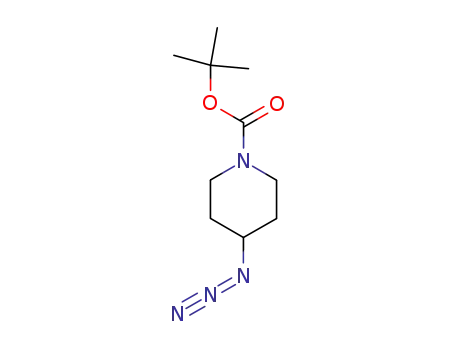
tert-butyl 4-azidopiperidine-1-carboxylate
-
206273-87-2
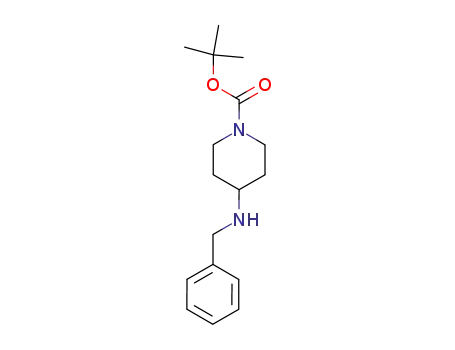
4-benzylamino-piperidine-1-carboxylic acid tert-butyl ester
-
79099-07-3

N-tert-butyloxycarbonylpiperidin-4-one
87120-72-7 Downstream products
-
87120-73-8
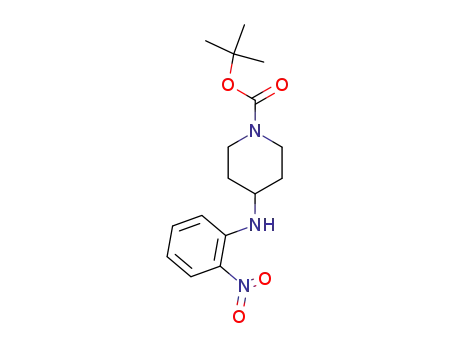
tert‐butyl 4‐((2‐nitrophenyl)amino)piperidine‐1‐carboxylate
-
87120-79-4
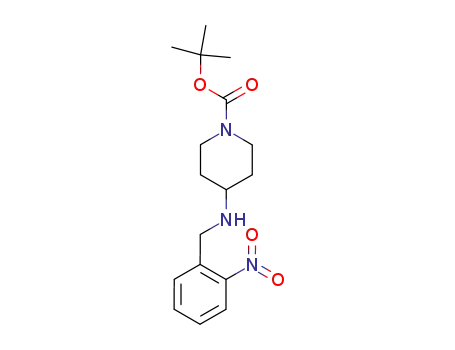
tert-butyl 4-((2-nitrobenzyl)amino)piperidine-1-carboxylate
-
198823-38-0
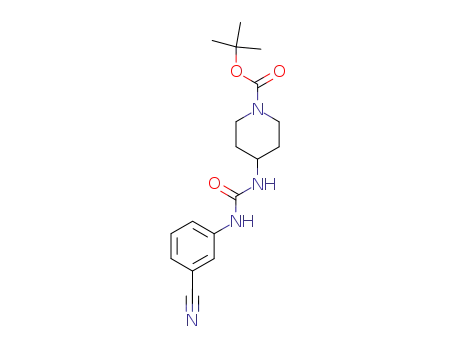
N-(3-cyanophenyl)-N'-(1-(t-butoxycarbonyl)piperidin-4-yl)urea
-
1233955-69-5
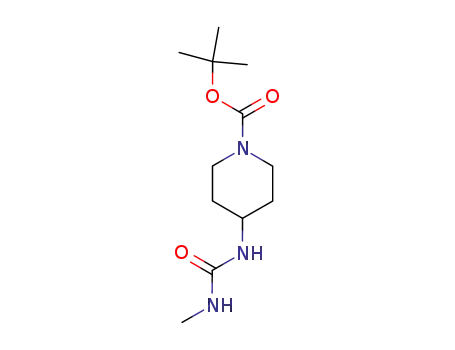
tert-butyl 4-[(methylcarbamoyl)amino]piperidine-1-carboxylate
Relevant Products
-
1-(4-Methylphenyl)-2-nitropropene
CAS:29816-55-5
-
4-PIPERIDINECARBOXYLIC ACID T-BUTYL ESTER HCL
CAS:892493-65-1
-
4-Chloro-N-methylpiperidine
CAS:5570-77-4